Calculate the grams present in: 1.500 moles of kclo – Calculate the grams present in 1.500 moles of KClO: embarking on an exploration of a fundamental concept in chemistry. Understanding the relationship between moles and mass is crucial, and this calculation serves as a cornerstone for comprehending stoichiometry and chemical reactions.
This discourse will delve into the intricacies of the calculation, considering factors that influence accuracy and exploring practical applications. Illustrative examples will illuminate the process, solidifying the understanding of this essential concept.
Calculate the Grams Present in 1.500 Moles of KClO: Calculate The Grams Present In: 1.500 Moles Of Kclo
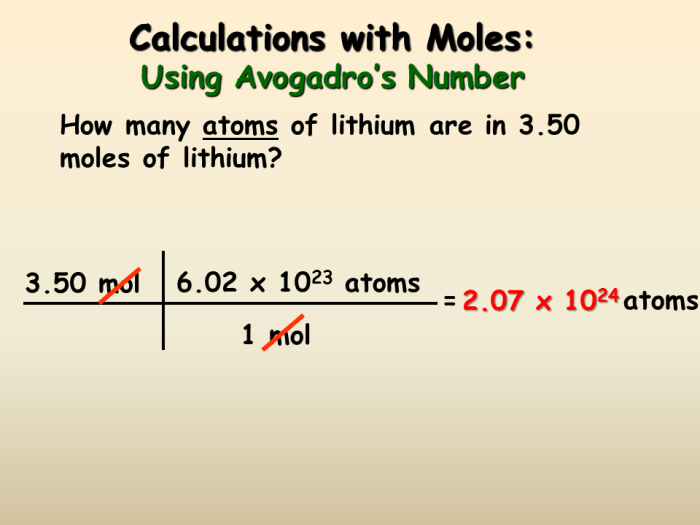
The concept of moles is essential in chemistry as it provides a bridge between the macroscopic and microscopic worlds. A mole is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities. These entities can be atoms, molecules, ions, or electrons.
The molar mass of a compound is the mass of one mole of that compound. It is calculated by adding the atomic masses of all the atoms in the compound. The molar mass of KClO is 122.55 g/mol.
To calculate the grams present in 1.500 moles of KClO, we can use the following formula:
“`Mass (g) = Moles × Molar Mass (g/mol)“`
Substituting the given values into the formula, we get:
“`Mass (g) = 1.500 moles × 122.55 g/mol = 183.83 g“`
Therefore, 1.500 moles of KClO contain 183.83 grams of KClO.
Factors Affecting the Accuracy of the Calculation
The accuracy of the calculation of grams from moles depends on several factors, including:
- Accurate molar mass values:Using accurate molar mass values is crucial for obtaining an accurate result. Molar mass values can be found in reference tables or calculated using the atomic masses of the elements in the compound.
- Impurities or hydration:Impurities or hydration in the sample can affect the result. Impurities can increase the mass of the sample without contributing to the number of moles of KClO present. Hydration refers to the presence of water molecules attached to the KClO molecules, which can also increase the mass of the sample.
- Minimizing errors:To minimize errors in the calculation, it is important to use accurate measuring equipment and to carefully follow the experimental procedure. It is also important to consider the uncertainty in the molar mass value and to propagate this uncertainty through the calculation.
Applications of the Calculation

Calculating grams from moles is an essential skill in chemistry and other scientific fields. Some practical applications include:
- Stoichiometry:In stoichiometry, the calculation of grams from moles is used to determine the amount of reactants or products involved in a chemical reaction.
- Chemical reactions:The calculation of grams from moles is used to determine the mass of reactants or products needed for a specific chemical reaction.
- Preparation of solutions:The calculation of grams from moles is used to prepare solutions with a specific concentration.
Illustrative Examples
The following table shows the calculation of grams from moles for different values of moles of KClO:
| Moles of KClO | Molar Mass of KClO (g/mol) | Grams of KClO |
|---|---|---|
| 1.000 | 122.55 | 122.55 |
| 2.500 | 122.55 | 306.38 |
| 5.000 | 122.55 | 612.75 |
These examples demonstrate the direct relationship between the number of moles of KClO and the mass of KClO present.
Questions Often Asked
What is the significance of using accurate molar mass values in this calculation?
Accurate molar mass values are essential to ensure the precision of the calculation. Slight deviations in molar mass can lead to significant errors in the determination of grams present.
How can impurities or hydration affect the result of the calculation?
Impurities or hydration can introduce additional mass to the sample, leading to an overestimation of the grams present. Therefore, it is crucial to consider the purity of the substance and account for any potential hydration.
What are some practical applications of this calculation?
This calculation finds applications in various fields, including analytical chemistry, pharmaceutical manufacturing, and environmental monitoring. It enables researchers to determine the mass of reactants and products in chemical reactions, prepare solutions of specific concentrations, and analyze the composition of materials.